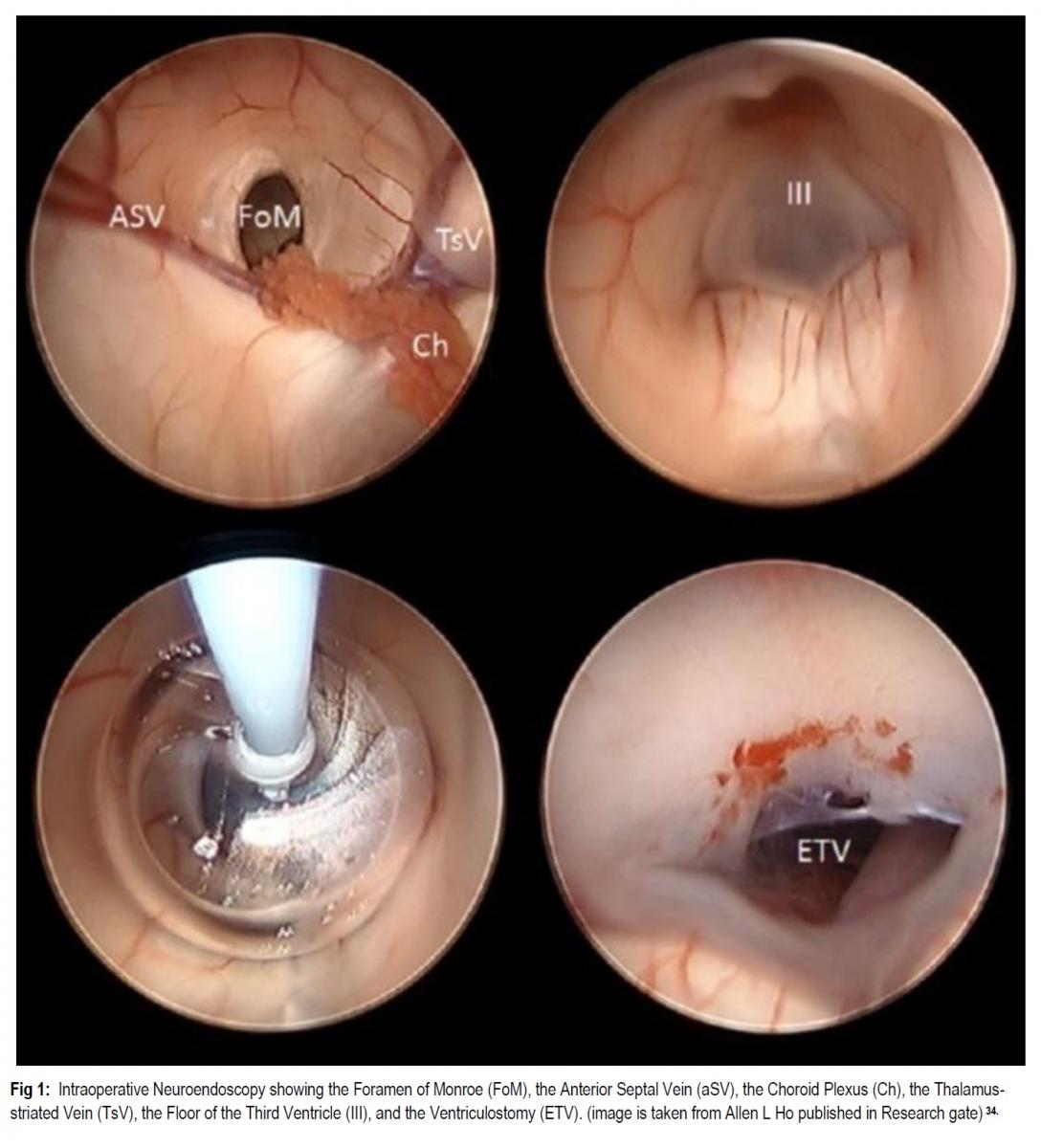
INTRODUCTION
Endoscopic third ventriculostomy (ETV) appears as an effective alternative to cerebrospinal fluid (CSF) shunt placement to treat obstructive hydrocephalus. The rationale for this procedure would be the restoration of the obstructed CSF passage through a ventriculocystostomy.1,2.
This is an attempt to avoid the high costs and complications associated with a CSF shunt.3-6 In 2005, an initial work by Warf 7 described higher success rates in selected groups when associating choroid plexus coagulation (CPC) with ETV (CPC/ETV). The effect provided by CPC would be to decrease CSF production, thus helping to restore CSF homeostasis.8 Driven by the encouraging results of this study, studies are being developed that identify prognostic factors that would influence the success rate of the procedure; mainly the age of the patient, the etiology of the hydrocephalus, the narrowing of the prepontine cistern, the previous CSF shunts, and the degree of coagulation of the plexus. 9-22.
The purpose of this review is to determine the prognostic factors that would influence the effectiveness rate of ETV/CPC in the control of hydrocephalus, as a contribution to decision-making, formation of expectations, and delivery of information to patients and/or caregivers.
ETV / CPC efficacy: current evidence
Study 7 that prompted the investigation of TVE / CPC showed that the association of TVE with CPC had a higher success rate in the control of hydrocephalus than isolated TVE (68% vs 54%, p = 0.0012). In children under 1 year, the effectiveness rate was 66% vs 80% in those over 1 year. When comparing by etiology in children under 1 year, the lowest effectiveness rate was 40% in post-hemorrhagic hydrocephalus, the highest was 76% in myelomeningocele.
The idea emerged from this study that some groups of patients would benefit more from the procedure. PubMed was searched for "Endoscopic Third Ventriculostomy" and "Choroid Plexus Cauterization". We included those studies in which the procedure was used together as a treatment for hydrocephalus and the results differentiated the efficacy of the procedure between groups according to some variable, or that the study was of a subpopulation in which a characteristic of the group studied was a variable comparable with those of other studies found according to the previous criterion.
In a cohort of 9 patients with congenital aqueduct stenosis, ETV / CPC had a success rate of 81.9%. A prospective study10 in patients with hydrocephalus associated with myelomeningocele showed a 76% effectiveness rate for ETV / CPC. In addition, it showed the scars of the prepontine and interpeduncular cisterns (p = 0.021) and of the choroid plexus (p = 0.026) as independent factors of treatment failure. The age and permeability of the aqueduct were not significant factors.
Another study 11 in patients with posthemorrhagic hydrocephalus associated with prematurity demonstrated a success rate of ETV / CPC of 40%. The success rate was 100% in those with an unobstructed prepontine cistern vs 14% in those with an obstructed cistern (p = 0.033). A 73% success rate of ETV / CPC is described in patients with hydrocephalus associated with the Dandy-Walker complex.12
In a group of patients in which ETV / CPC showed an overall success rate of 50%, the etiology, and age at the time of surgery were not associated with the outcome. The use of a flexible endoscope achieved> 90% CPC in 88% vs 14% with the use of a rigid endoscope (p <0.001). The success of the procedure was greater in those with ≥90% CPC (82% vs 36%, p = 0.0501). 13
A prospective series 14 showed a 57% success rate for ETV / CPC as the only procedure and a 65% independence of derivative. Independent failure factors included age less than 6 months, postinfectious etiology, cistern scars, and a previous referral. A cohort15 demonstrated an overall CPC / ETV success of 43%. The mean age of the patients in which the procedure was successful was higher (3.9 months vs 0.8 months, p = 0.01).
A 37% success rate of ETV / CPC is reported in patients with posthemorrhagic hydrocephalus of prematurity. A narrow prepontine cistern documented by magnetic resonance imaging (MRI), and a corrected age of fewer than 0 weeks at the time of surgery were independent factors of failure.16 In contrast to previous findings, one study describes that a scarred cistern seen endoscopically was associated with failure of the ETV / CPC, however, the degree of CPC and the imaging findings of the anatomy of the cistern prior to surgery was not associated with failure. 17
Another study with hydrocephalus of various etiologies showed an overall success rate of the procedure of 75%, with the best success rate in myelomeningocele (87.5%) and the worst in postinfectious disease (50%).18 In a multivariate analysis of prospective results, it was determined that younger age (p = 0.002), larger ventricular size (p = 0.009), and lower grade of CPC (p = 0.02) were associated with failure of the ETV / CPC. 19
A prospective study analyzed predictive factors for the success of ETV / CPC. The overall success rate was 48% at 6 months. As a single variable, age (p = 0.026) and etiology (p = 0.001) were associated with the outcome. Multivariate analysis showed that a corrected age <1 month (adjusted RR 1.9, 95% CI 1.0–3.6) and intraventricular hemorrhage associated with prematurity (adjusted RR 2.0, 95% CI 1.1–3.6) were independent factors of failure. A larger preoperative ventricular size was also associated with greater failure (RR 6.9, 95% CI 1.3–37.9; p = 0.027). The use of a flexible endoscope (p = 0.095), formal training in this procedure (p = 0.089) and a CPC degree> 90% (p = 0.94) had no association with the outcome. 20
Haitian Experience 21 shows a global ETV / CPC success of 52.2%. In addition, a modified lower Endoscopic Third Ventriculostomy Success Score 22 was associated as an independent risk factor for failure (RR 0.072, 95% CI 0.016-0.32, p <0.001).
In one study 23 with a 56% overall success rate of ETV / CPC, patients with myelomeningocele (83%) and congenital aqueductal stenosis (83%) were the groups with the highest success rate, on the contrary, those with hemorrhage intraventricular were the ones with the lowest success rate (40%). The appearance of CSF turbulence in rapid sequence MRI was more frequent in the group of patients in which the ETV / CPC failed (55% vs 18%, p = 0.02). The sensitivity and specificity of CSF turbulence as a radiographic marker of ETV / CPC failure were 80% and 58%, respectively. Choroid plexus persistence on imaging was recorded in 71% of patients who required subsequent bypass, compared with 6% of patients who did not require CSF bypass (p = 0.0001). Visualization of the choroid plexus in images despite ETV / CPC reflected treatment failure with 91% sensitivity and 81% specificity.
Study 24 compared the effectiveness of the use of a rigid endoscope versus a flexible endoscope when performing an ETV / CPC. In the group in which a flexible endoscope was used, CPC of the bilateral temporal horn was achieved in 98.9% vs 47.1% in the rigid endoscope group (p <0.001). The 12-month success rate according to survival analysis was 57% with a flexible endoscope vs 38% with a rigid endoscope (p = 0.0044). In the unadjusted survival analysis, rigid Neuroendoscopy was associated with failure (RR 1.61, 95% CI 1.05–2.48, p = 0.031). The analysis adjusted for covariates yielded a RR for rigid versus flexible Neuroendoscopy failure of 1.1 (95% CI 0.69–1.73, p = 0.70). According to etiology, the highest risk of failure was associated with postinfectious etiology (RR 3.48, 95% CI 1.73–7.01, p = 0.0027), while the group with myelomeningocele demonstrated the lowest risk of failure (RR 0.49, 95% CI 0.25– 0.94, p = 0.019). The presence of any type of previous CSF shunt (RR 1.78, 95% CI 1.15-2.77, p = 0.013) and the presence of a scarred prepontine cistern (RR 2.80, 95% CI 1.77-4.43, p <0.000) were also predictors of failure. Age greater than the median of 3.2 months was associated with a lower failure rate (RR 0.58, 95% CI 0.38–0.90, p = 0.014). Sex was not significantly associated with any type of outcome.
DISCUSIÓN
In the last decade, more evidence has accumulated than ever regarding the efficacy and safety of ETV / CPC as a procedure to treat hydrocephalus. Initially, the studies were in Africa to find a viable alternative for CSF shunt, due to its significant profile of complications 5 that include infections, ventricular peritoneal shunt (VPS) dysfunction, and over drainage, among others, in an environment where that patient follow-up was not easy. Encouraging results led the researchers to replicate the experience in North America, where the economic barrier is not the greatest difficulty.
In this work, the classical theory of CSF circulation will be given as an explanation of the clinical results. However, it is important to note that, under the new hydrodynamic theory,25 which argues that CSF would be produced throughout the entire central nervous system through a balance between hydrostatic and oncotic forces like the rest of the body fluids, this procedure would not be solving the pathophysiology of the condition. The rationale for this procedure is to restore CSF homeostasis by achieving a balance between CSF production and absorption. On the one hand, the ETV would solve an obstructive factor possibly involved in the reduction of CSF absorption. On the other hand, the CPC aims to eliminate a source of CSF production that, although not the largest,26-28 has an influence on the total volume of CSF and, by not being present, contributes to its stabilization.
Accumulated evidence shows us that adding CPC to ETV would increase the overall success rate compared to ETV alone.7,9,29 The patients with higher success rates according to etiology were patients with hydrocephalus secondary to myelomeningocele, congenital aqueductal stenosis, and Dandy-Walker complex.7,9,10,12 The patients with the lowest success rates were those with postinfectious hydrocephalus secondary to an intraventricular hemorrhage of prematurity.11,14,16,18,20,23,25 The disparity in the success rate according to etiology is explained by pathophysiological reasoning. In cases where there is a probable obstruction in the flow of CSF, such as myelomeningocele,30 the ETV would act as a “bypass” to the obstruction, together with the respective role of the CPC in reducing the production of CSF. On the other hand, in cases in which there is no clear obstructive factor, the ETV would not have a greater influence, so the CPC would become the main mechanism by which the CSF volume would stabilize, being this suboptimal by itself.
Age was also cross-sectionally classified as a prognostic factor. Early ages, at the time of surgery, were proportionally associated with higher rates of ETV / CPC failure, this effect was maximum in patients under 1 month of corrected age.7,14-16,19,20 One reason for The fact that this difference exists according to the age of the patient may be because at younger ages the floor of the third ventricle is thicker, so the procedure itself can be frustrating or in the long run have a greater probability of closing the stoma.31,32 It could also be being due to a more distensible skull at a younger age, this would more easily allow plastic deformation of the skull leading to the diagnosis of failure of the procedure; In contrast, a skull without this plasticity would determine a brain with lower “compliance”, with greater increases in pressure that, even though they are subclinical, may have a role in CSF homeostasis, by altering its hydrodynamics, as a brake on its subsequent development of hydrocephalus.
Another prognostic factor for failure that was reproduced throughout the studies was the presence of a narrow prepontine cistern on preoperative imaging studies or during the procedure.10,11,14,16,17,24 This is explained because having an obstruction in the CSF circuit in the compartment that connects with the third ventricle through the stoma, would not fulfill its purpose. The failure rate also increases if, in some centers during ventriculoscopy a significant anatomical distortion is identified, the procedure is abandoned and assumed as a failure. Studies in which a narrow prepontine cistern was not associated with failure could be due to inherent variability between observers when evaluating neuroimaging. 17.20
The presence of CSF shunt prior to performing ETV / CPC was also classified as a risk factor for failure of the latter.14,24 Likewise, larger ventricles at the time of surgery were predictors of failure.20 The reason for which patients with previous bypass procedures and larger ventricles had a lower success rate maybe because they were patients with a more advanced pathological process, whose result would be insufficient, regardless of the procedure used. In contrast, patients with smaller ventricles could be more successful due to an arrested pathophysiological process.
The degree of cauterization of the choroid plexus was proportionally associated with higher success rates of the joint procedure, being a predictor of failure in cases where it was less than 90%.13,19 It is even added to the revised ETV Success Score.22 Likewise, the use of a flexible endoscope is associated with a higher degree of CPC and also a higher success rate of ETV / CPC compared to the use of a rigid endoscope.24 However, there are also results that would indicate that there is no significant association between the extension of the cauterization of the plexus and the success rate of the procedure.17,20 From a pathophysiological point of view, it would make sense that a greater extension of the cauterization results in a greater decrease in the production of CSF in the plexus, thus contributing more to the control of hydrocephalus. It is described that not only is the coagulation of the entire plexus important, but also of the vessels that run through it, including the branches of the anterior choroid artery, the posterior lateral choroidal artery, and the superior choroidal vein.7,29,33 The difference in results between studies could be partly due to the lack of a standardized method to report the degree of CPC, or due to differences in surgical technique. Therefore, prospective studies are needed that use the same system to report the degree of CPC and standardization of the technique before cataloging the degree of CPC above or below 90% as an independent factor for the outcome of the procedure. Lower scores in the revised ETV Success Score were independently associated with ETV / CPC failure, which indirectly supports the idea that the degree of cauterization does influence the outcome. 21
The use of radiographic markers as a prognosis for the success of the procedure was also investigated. CSF turbulence and the presence of choroid plexus on posterior rapid sequence MRI after an ETV / CPC were associated with lower success rates. The turbulence would be present due to a possible obstructive factor not resolved with the ETV or another factor that is altering the hydrodynamics of the CSF, so the pathophysiological process would not have been resolved. On the other hand, the presence of CPC in an imaging examination would indicate that the procedure to decrease the amount of CSF produced was not completely achieved, so it would have less effect in trying to stabilize the volume of CSF. In any case, it would be appropriate to generate formal studies that assess the intra- and interobserver reliability of these findings to fully corroborate them. 23
Short-term outcomes have been studied and indicate that adding CPC to ETV is a safe and effective practice in increasing the success rate of ETV as a treatment for hydrocephalus. However, most of these studies lack a long follow-up and analysis of the neurocognitive and functional outcomes of the patients. Within these, it would be prudent to include data on the satisfaction and quality of life of the patient and her caregivers, which within the practice of pediatric neurosurgery is a core issue when making decisions. Therefore, new randomized clinical trials are required that directly compare ETV / CPC with CSF shunts, with a longer follow-up, standardizing definitions of procedural failure, and that evaluate neurodevelopmental outcomes to determine in which cases the patients would benefit. patients of one procedure over the other as a new standard of care. Until the evidence described is developed, the decision to use ETV / CPC should be discussed on a case-by-case basis, weighing the many factors that influence its success, as well as the social health context and the informed decision of the patient's family.
CONCLUSION
Short-term outcomes have been studied and indicate that adding CPC to ETV is a safe and effective practice in increasing the success rate of ETV as a treatment for hydrocephalus. Furthermore, the overall success rate of the joint procedure makes it a reasonable first option for hydrocephalus in selected patients.
Factors associated with a higher success rate of ETV / CPC are Hydrocephalus secondary to myelomeningocele, congenital aqueductal stenosis, and Dandy-Walker Complex; older ages (mainly> 1 year); and probably cauterization ≥90% of the choroid plexus.
Negative prognostic factors include corrected ages younger at the time of the procedure, with the maximum effect in the group <1 month; previous CSF bypass procedure; posthemorrhagic and postinfectious etiology; narrow prepontine cistern on neuroimaging or ventriculoscopy; the presence of CSF turbulence and visualization of postoperative choroid plexus in rapid sequence MRI; and probably cauterization <90% of the choroid plexus. Randomized clinical trials that compare ETV / CPC directly with CSF shunt therapies, and that study long-term neurocognitive and quality of life outcomes, are still needed.
REFERENCES
-
Idowu O, Doherty A, Tiamiyu O. Initial experience with endoscopic third ventriculostomy in Nigeria, West Africa. Childs Nerv Syst. 2008; 24:253–255.
-
Zandian A, Haffner M, Johnson J, Rozzelle CJ, Tubbs RS, Loukas M. Endoscopic third ventriculostomy with/without choroid plexus cauterization for hydrocephalus due to hemorrhage, infection, Dandy-Walker malformation, and neural tube defect: a meta-analysis. Childs Nerv Syst. 2014;30(4):571‐578.
-
Drake JM, Kestle JR, Milner R et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery. 1998; 43:294–303.
-
Mugamba J, Stagno V. Indication for endoscopic third ventriculostomy. World Neurosurg. 2013; 79(2 Suppl): S20.e19‐S20.e23.
-
Wu Y, Green NL, Wrensch MR, et al. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery. 2007;61(3):557-563.
-
Malheiros JA, Trivelato FP, Oliveira MM, et al. Endoscopic choroid plexus cauterization versus ventriculoperitoneal shunt for hydranencephaly and near hydranencephaly: a prospective study. Neurosurgery. 2010; 66:459–464; discussion 464.
-
Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg. 2005; 103: 475-481.
-
Morota N, Fujiyama Y. Endoscopic coagulation of choroid plexus as a treatment for hydrocephalus: indication and surgical technique. Childs Nerv Syst. 2004; 20:816–820.
-
Warf BC, Tracy S, Mugamba J. Long-term outcome for endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization for congenital aqueductal stenosis in African infants. J Neurosurg Pediatr. 2012; 10: 108-111.
-
Warf BC, Campbell JW. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment of hydrocephalus for infants with myelomeningocele: long-term results of a prospective intent-to-treat study in 115 East African infants. J Neurosurg Pediatr. 2008; 2:310-316.
-
Warf BC, Campbell JW, Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst. 2011;27(7):1063‐1071.
-
Warf BC, Dewan M, Mugamba J. Management of Dandy-Walker complex-associated infant hydrocephalus by combined endoscopic third ventriculostomy and choroid plexus cauterization. J Neurosurg Pediatr. 2011; 8:377-383.
-
Kulkarni AV, Riva-Cambrin J, Browd SR, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective Hydrocephalus Clinical Research Network study. J Neurosurg Pediatr. 2014;14(3):224‐229.
-
Stone SS, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J Neurosurg Pediatr. 2014; 14:439–446.
-
Dewan MC, Lim J, Morgan CD, et al. Endoscopic third ventriculostomy with choroid plexus cauterization outcome: distinguishing success from failure. J Neurosurg Pediatr. 2016;25(6):655‐662.
-
Chamiraju P, Bhatia S, Sandberg DI, Ragheb J: Endoscopic third ventriculostomy and choroid plexus cauterization in posthemorrhagic hydrocephalus of prematurity. J Neurosurg Pediatr. 2014; 13:433–439.
-
Weil AG, Fallah A, Chamiraju P, et al. Endoscopic third ventriculostomy and choroid plexus cauterization with a rigid neuroendoscope in infants with hydrocephalus. J Neurosurg Pediatr. 2016;17(2):163‐173.
-
Bankole OB, Ojo OA, Nnadi MN, et al. Early outcome of combined endoscopic third ventriculostomy and choroid plexus cauterization in childhood hydrocephalus. J Neurosurg Pediatr. 2015; 15:524–528.
-
Kulkarni AV, Riva-Cambrin J, Rozzelle CJ, et al. Endoscopic third ventriculostomy and choroid plexus cauterization in infant hydrocephalus: a prospective study by the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 2018;21(3):214‐223.
-
Riva-Cambrin J, Kestle JRW, Rozzelle CJ, et al. Predictors of success for combined endoscopic third ventriculostomy and choroid plexus cauterization in a North American setting: A Hydrocephalus Clinical Research Network study [published online ahead of print, 2019 May 31]. J Neurosurg Pediatr. 2019;1‐11.
-
Shah AH, LaFortune Y, Ibrahim GM, et al. Endoscopic third ventriculostomy with choroid plexus cauterization for the treatment of infantile hydrocephalus in Haiti [published online ahead of print, 2020 Jan 10]. J Neurosurg Pediatr. 2020;1‐6.
-
Warf BC, Mugamba J, Kulkarni AV: Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus in Uganda: report of a scoring system that predicts success. J Neurosurg Pediatr. 2010; 5:143–148.
-
Pindrik J, Rocque BG, Arynchyna AA, et al. Radiographic markers of clinical outcomes after endoscopic third ventriculostomy with choroid plexus cauterization: cerebrospinal fluid turbulence and choroid plexus visualization. J Neurosurg Pediatr. 2016; 13:1–9.
-
Wang S, Stone S, Weil AG, et al. Comparative effectiveness of flexible versus rigid neuroendoscopy for endoscopic third ventriculostomy and choroid plexus cauterization: a propensity score-matched cohort and survival analysis. J Neurosurg Pediatr. 2017;19(5):585‐591.
-
Orešković D, Klarica M. Development of hydrocephalus and classical hypothesis of cerebrospinal fluid hydrodynamics: facts and illusions. Prog Neurobiol. 2011;94(3):238‐258.
-
Milhorat TH, Hammock MK, Chien T, Davis DA. A normal rate of cerebrospinal fluid formation five years after bilateral choroid plexectomy. Case report. J Neurosurg. 1976;44(6):735-739.
-
Pollay M, Curl F. Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol. 1967;213(4):1031-1038.
-
Sato O, Bering EA. Extra-ventricular formation of cerebrospinal fluid. No to Shinkei. 1967;19(9):883-885.
-
Zandian A, Haffner M, Johnson J, et al. Endoscopic third ventriculostomy with/without choroid plexus cauterization for hydrocephalus due to hemorrhage, infection, Dandy-Walker malformation, and neural tube defect: a meta-analysis. Childs Nerv Syst. 2014;30(4):571‐578.
-
Cohen AR, Robinson S. Early management of myelomeningocele, in McLone DG (ed): Pediatric Neurosurgery, ed 4. Philadelphia: WB Saunders, 2001, pp 241–260.
-
Tubbs RS, Hattab EM, Loukas M, et al. Histological analysis of the third ventricle floor in hydrocephalic and non-hydrocephalic brains: application to neuroendocrine complications following third ventriculostomy procedures. J Neurosurg Pediatr. 2012; 9:178–181.
-
Sufianov AA, Sufianova GZ, Iakimov IA. Endoscopic third ventriculostomy in patients younger than 2 years: outcome analysis of 41 hydrocephalus cases. J Neurosurg Pediatr. 2010;5: 392–401.
-
Hallaert GG, Vanhauwaert DJ, Logghe K, et al. Endoscopic coagulation of choroid plexus hyperplasia. J Neurosurg Pediatr. 2012;9(2):169‐177.
-
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Authors Contributions
Conception and design: Smoquina, Zulueta. Drafting the article: Smoquina. Critically revising the article: Smoquina. Reviewed submitted version of manuscript: Smoquina, Zulueta. Approved the final version of the manuscript on behalf of all authors: Smoquina.
Correspondence